Research Projects-Opportunities for Undergraduate Students
I consider myself to be a molecular engineer: I design, synthesize and characterize compounds that I hope will have specific useful properties and then test how well these compounds do the work I’ve designed them to do.
I am interested in compounds with two types of properties in particular:
- the ability to catalyze particular types of redox reactions, and
- the ability to absorb, store and convert visible light to other forms of energy
The projects listed and briefly described below are only some of those I have available for undergraduate students to work on. All of these projects are designed to incorporate organic, inorganic, analytical, physical and computational chemistry concepts and techniques so that students can learn how these fields are all connected and all necessary in molecular design.
1. 2-(2-Hydroxyphenyl)-substituted Benzimidazoles as Ligands
2-(2-Hydroxyphenyl) benzothiazolate and 8-hydroxyquinolate metal complexes have been studied as emitting materials in electroluminescent devices, e.g. light-emitting diodes. Metal complexes of 2-(2-hydroxyphenyl) benzimidazole complexes have not been investigated to nearly the same degree. Thus it seems reasonable to explore this class of ligands.
I have shown the structure of one target ligand here along with the way it can act as a bidentate ligand to metal ions.
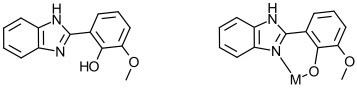
Can we synthesize ligands where different substituents on the phenolate ring and/or the fused benzene ring, tune the photophysical properties of metal complexes of these ligands?
2. Complexes of Ligands Based on the 1,8-Naphthyridine Template
Complexes of substituted 1,8-naphthyridines have been studied to see whether their electronic properties can lead to interesting photophysical behavior. An example of a substituted 1,8-naphthyridine and its mode of binding to a metal ion are shown below.
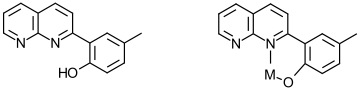
My interests lie in expanding the substitution pattern in the phenolate ring to see (as in Project #1 above) whether this affects the electronic properties of metal complexes (Ru(II) for example) containing one or more of these ligands.
A related project involves the characterization of ligands of the formyl-substituted ligand shown here along with the Schiff base product with a generic amine RNH2.
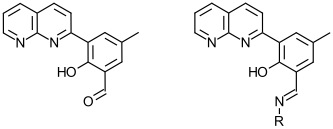
Can we make new tetradentate ligands that combine in one molecule the structural and electronic properties of naphthyridines and Schiff bases?
3. Water-Soluble and Unsymmetrical Schiff Bases Complexes
The prototypical tetradentate Schiff base ‘salen’ has been known for about 150 years, but we can still teach this old dog new tricks! For example it is known that metal complexes of this type of ligand can act as effective catalysts for a variety of organic oxidations.
My own interests lie in the study of two related systems, shown below.
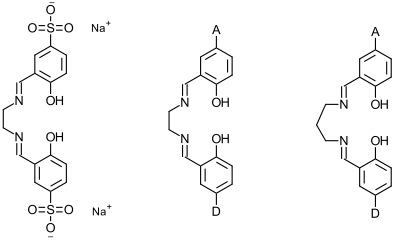
The molecule on the left is a sulfonated version of the ‘salen’ ligand. Metal complexes of this ligand should be more water-soluble than those of ‘salen’ itself. Since water is an environmentally ‘green’ solvent for chemical reactions I am interested in investigating the catalytic activities of metal complexes of this and related ligands. In addition I am interested in investigating the possibility of using complexes of these ligands as phase-transfer catalysts, by swapping the sodium ions with more hydrophobic quaternary ammonium cations (NR4+).
The other two ligands are unsymmetrical versions of ‘salen’ and the related ‘saltn’ ligand in which ‘A’ and ‘B’ represent different substituents. It is difficult to synthesize unsymmetrical ‘salens’ but one procedure for making copper(II) complexes has been published. I hope to repeat and extend this work by making the copper(II) complexes, removing the Cu2+ ion and replacing it with other metal ions such as Mn3+, Fe3+ or Zn2+. The manganese and iron complexes may be redox catalysts; the zinc complexes may have useful fluorescent properties.
If any of these projects interest you, or if you’d like to find out what other projects I have available, please feel free to ask me about them.

